Combustion reactions are almost always exothermic ie they give off heat. Combustion is a chemical change.

Combustion Reaction Definition Characteristics Examples
The combustion reactions undergone by methane are also known to yield water and carbon dioxide as products.

. Which of the following are combustion reactions. B It is the combustion of ethanol with the formation of carbon dioxide and water. Apologia Physics Module 9.
There are several types of combustion reactions in chemistry with the two most common being complete and incomplete combustion. When wood burns in the presence of oxygen in what form is energy released. The first step is to write the skeleton equation ensuring the formula of.
1 CH 0 -0000 H2O 1 2 CO CO - Caco a 3 Pbco -- PbO 8 CO G CHOH 06 -CO0 H20 1 D 2 3 and 4 E 3 and 4 A 1 and 4 B 123 and 4 C 13 and 4 13 3 p. Petrol consists of different hydrocarbons while burning they react with oxygen forming carbon dioxide and water. The reaction between a HC hydrocarbon and O oxygen to produce CO2 carbon dioxide and H2O water is a standard form of a combustion reaction.
It is the combustion of gas Methane CH4 when it encounters oxygen 2O2. Any chemical reaction that involves oxygen as one of the. Burning petrol is a combustion reaction.
Which of the following is a Combustion reaction. Which of the following is NOT a combustion reaction. In grills fireplaces etc.
1 CH4 g O2 g CO2 g H2O l 2 CaO s CO2 g CaCO3 s 3 PbCO3 s PbO s CO2 g 4 CH3OH l O2 g CO2 g H2O l A 1 and 4 B 1 2 3 and 4 C 1 3 and 4 D 2 3 and 4 E 3 and 4. 2 ZnS s 3 O2 g---- 2 ZnO s 2 SO2 g For each of the following chemical reactions write a word equation a skeleton equation and a balanced chemical equation. Reaction 1 CH4 O2 ----- CO2 H2O Reaction 4 CH3.
417 Chemistry Unit Assessment K12. Combustion reactions always involve molecular oxygen O2. The chemical equation for this reaction is given by.
Exothermic is the process or reaction that releases energy in the form of heat. When wood burns it must do so in the presence of O2. NOgO3gNO2gO2g Combustion reactions release large amounts of energy as heat andor light.
The process of balancing combustion reactions may seem daunting but is easy if the following steps are followed. View the full answer. The products are solid zinc oxide and sulfur dioxide gas.
The combustion of methanol sometimes referred to as wood alcohol involves a chemical reaction between methanol and oxygen. Methane will burn forming C02 gas and H20 gas. A 1-pentene b 23-dimethyl-1-pentyne c 233-trimethyl-1-butyne d 23-dimethyl-1-heptene Answer.
In lighters the reaction of butane combustion is commonly seen. Here 2C4H10g reacts with 13O2g and after combustion gives 8CO2g 10H2O g as the resultant. The type or reaction varies depending on several factors such as the.
In a combustion reaction energy is released along with the formation of C O 2 Let have a look at the options. The correct IUPAC name for the molecule below is ___. Type of combustible material.
Anytime anything burns in the usual sense it is a combustion reaction. And a lot of heat is produced. Combustion refers to a high-energy chemical reaction in which fuel is oxidized and converted into a mixture of often gaseous products.
A It is combustion of Methane where carbon dioxide and water are formed. Be sure to show the state of each reactant and product. The next step of refining zinc involves heating the zinc.
A oxygen energy. Surface area of the material. C It is the complete combustion of carbon monoxide to form carbon dioxide.
Combustion is an exothermic reaction in that it involves the release of energy in the form of light and heatThe most common oxidizing agent in combustion reactions is atmospheric oxygen O but other oxidizing agents include. Which of the following is a combustion reaction. Combustion of propane takes place.
2CH3OH 3O2 4H2O 2CO2. FF1 test prep 4. It is a high-temperature exothermic reaction.
See what the community says and unlock a badge. In this 6CO2g 8H2O g is produced after the combustion between 2C3H8g and 7O2g. In the following reactions identify the physical states of the products and reactants and indicate A.
The following is a general combustion reaction. Thereforethe correct answer would be A. Sets found in the same folder.
EqFuel_sl or g excess O_2g rightarrow CO_2g H_2O_g eq Note that excess oxygen is in the formula. 4Fe 3O2 ---- 2Fe2O3. What must methane be combined with to undergo a Combustion reaction.
PetrolO 2 CO 2 H 2 O Heat. Which of the following generalized equations best represents a combustion reaction. A Hydration b Combustion c Hydrogeneation d Halogenation Answer.
12 e Which of the following are combustion reactions.
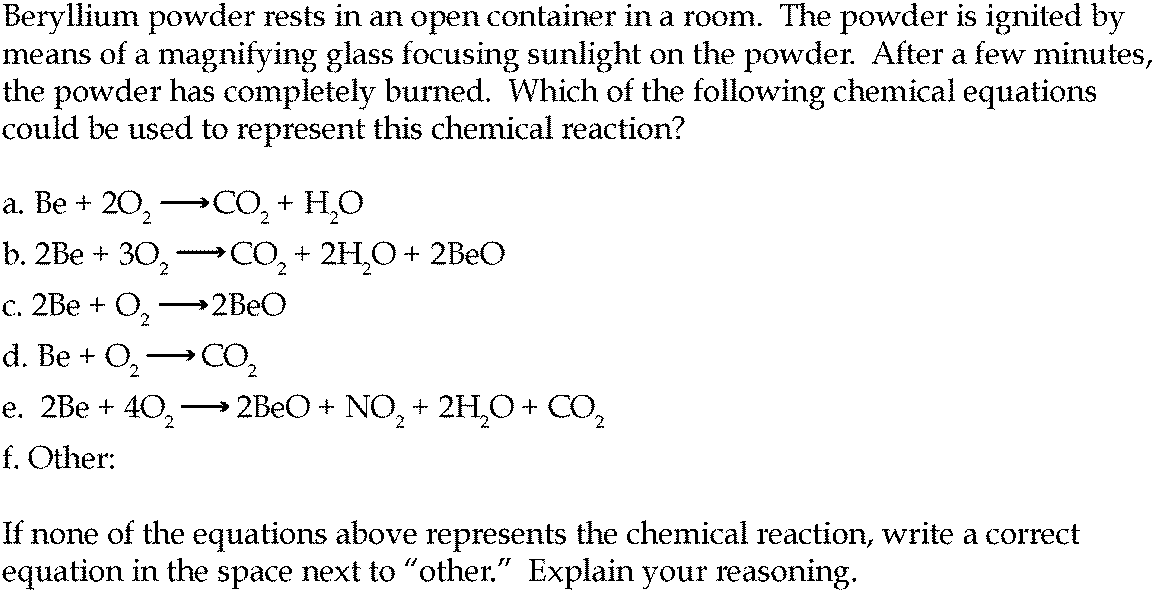
Combustion Always Produces Carbon Dioxide And Water A Discussion Of University Chemistry Students Use Of Rules In Place Of Principles Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C4rp00089g

Chapter 11 Combustion Updated 5 31 10

0 Comments